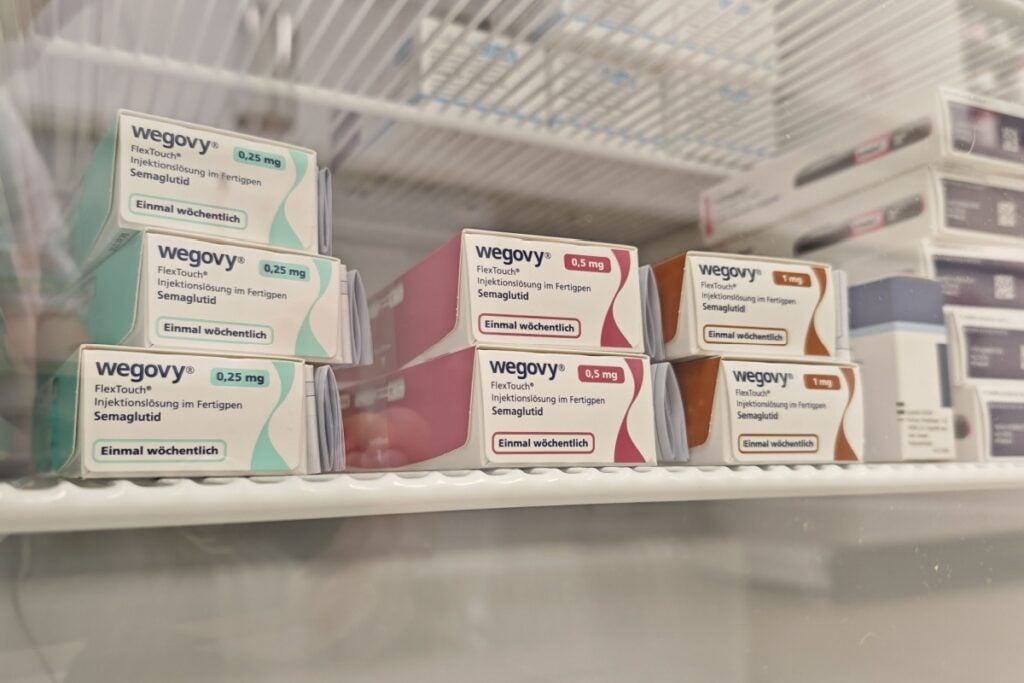
U.S. regulators have approved a once-daily tablet form of Wegovy, making it the first oral medication cleared in the United States specifically to treat obesity. The decision from the U.S. Food and Drug Administration gives Novo Nordisk a head start over Eli Lilly and Co., whose obesity pill candidate orforglipron is still under FDA review. Clinicians say an oral option could bring more patients into treatment, particularly those who avoid injections, while payers and employers weigh coverage decisions.
The approval comes after several years in which injectable GLP-1 medicines became mainstream. Novo Nordisk’s injectable Wegovy and Lilly’s Zepbound are widely prescribed, and the AP report notes that obesity affects roughly 100 million people in the United States. At the same time, access has been constrained by high prices and inconsistent insurance coverage, leaving a large gap between public interest and actual use.
How The Tablet Is Formulated And Taken
The pill contains 25 milligrams of semaglutide, the same active ingredient used in injectable Wegovy and diabetes drug Ozempic. Novo Nordisk previously brought a lower-dose semaglutide tablet, Rybelsus, to market for diabetes after FDA clearance in 2019. Like other GLP-1 drugs, semaglutide mimics a naturally occurring hormone involved in appetite regulation and satiety, helping many patients reduce hunger and feel full with less food.
Because the digestive tract can break down peptide medicines, Novo Nordisk said it designed the tablet with an added component intended to protect the drug long enough for absorption. That engineering comes with a strict routine: the company says the pill should be taken in the morning with a small sip of water on an empty stomach, followed by a 30-minute pause before eating or drinking. Lilly’s orforglipron, which the AP report says is being considered under an FDA program intended to speed some drug approvals, has been tested without comparable dosing restrictions.
Trial Results Highlight Effectiveness, Cost Remains Key
Evidence summarized in the AP report suggests the tablet can deliver weight loss close to the injectable benchmark. In a study published in The New England Journal of Medicine, participants taking oral Wegovy lost an average of 13.6% of body weight over about 15 months, compared with 2.2% for those on placebo. The AP report said injectable Wegovy has produced average weight loss of roughly 15% in studies, placing the pill’s results in a similar range.
Lilly’s trial data, also published in NEJM, showed participants on the highest tested dose of orforglipron lost 11.2% of body weight over nearly 17 months, versus 2.1% with placebo. Both oral drugs produced less weight loss than results reported for Lilly’s injectable Zepbound (tirzepatide), which targets GLP-1 and GIP and has been associated with about 21% average weight loss in research cited by the AP. Across the class, the most common side effects remain gastrointestinal symptoms such as nausea and diarrhea.
Novo Nordisk and obesity specialists argue that choice of format matters: some patients prefer a weekly injection, while others want a daily pill even if it requires timing rules. One trial participant, Chris Mertens, a 35-year-old physician in Wisconsin, told the AP he lost about 40 pounds on the pill during a Novo Nordisk study that began in 2022, and he described fewer persistent thoughts about food.
Pricing, however, is likely to determine how much the new option changes real-world access. The AP report said GLP-1 drugs can cost upward of 1,000 US-Dollar per month. Novo Nordisk said the pill’s starting dose would be offered for 149 US-Dollar per month through some providers, with additional pricing details expected in January.
The approval also lands amid sustained public health pressure. CDC estimates show adult obesity prevalence was 40.3% during August 2021–August 2023. A KFF Health Tracking Poll in May 2024 found about 12% of U.S. adults said they had ever taken a GLP-1 drug, suggesting demand will remain strong if costs fall and coverage expands.